HERBAL
MEDICINAL
PLANT

-organic---bulk-quantity-(2g-9500-seeds)_1.jpg)
COLTSFOOT
Tussilago farfara L.
(Asteraceae/Compositae) +
BY
RETTODWIKART THENU
-------------
COLTSFOOT
(koeltz’
fut)
Tussilago
farfara L. (Asteraceae/Compositae) +
SUMMARY AND PHARMACEUTICAL COMMENT
The
majority of the traditional uses associated with coltsfoot can be attributed to
the mucilage content. However, coltsfoot also contains toxic pyrrolizidine
alkaloids albeit at a low concentration. The risk of exposure to low
concentrations of unsaturated pyrrolizidine alkaloids is unclear although hepatotoxicity
following prolonged exposure has been documented (see Comfrey). The regular or excessive consumption
of coltsfoot, especially in the form of herbal teas, should therefore be
avoided.
TRADE NAMES
British
Tobacco, Bullsfoot, Butterbur, Rower Velure,
Foal's-Foot, Horse-Foot, Horsehoof, Ass's Foot, Foalswort
OTHER COMMON NAMES
Coughwort, Donnhove, Farfara,
Fieldhove, Filiusante Patrem, Flower Velure, Hallfoot, Kuandong Hua, Pas Diane
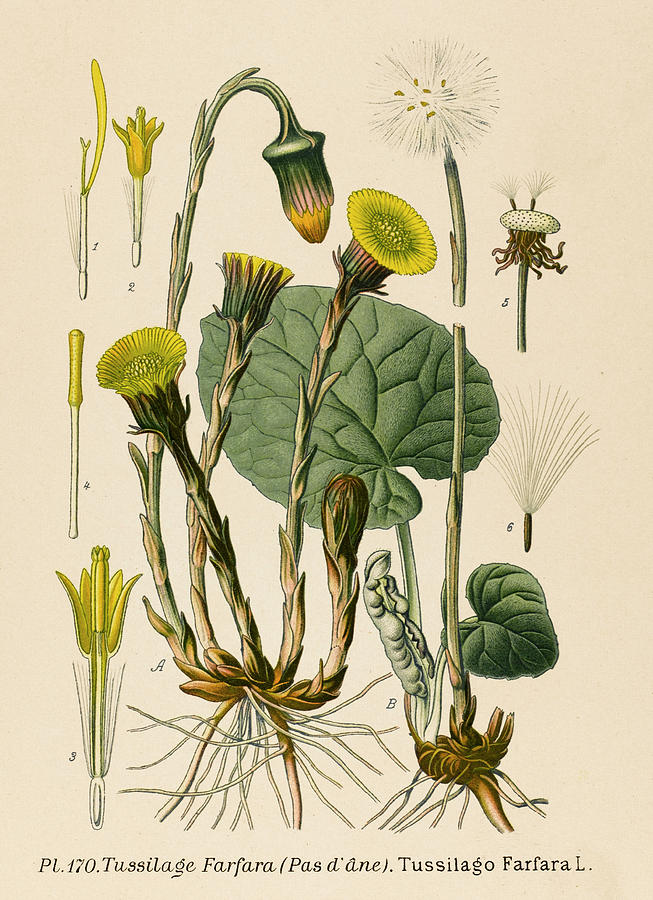
DESCRIPTION
MEDICINAL PARTS: The medicinal
parts are the dried inflorescences, the dried leaves and the fresh leaves.
FLOWER AND FRUIT: The yellow
compound flowers are in small, solitary capitula at the end of the scapes. The
lateral florets are lingual, narrow and female. The disc florets are tubular-campanulate,
5-petalled and male. The involucral bracts are almost as long,
linear-lanceolate and have a scarious margin. The fruit is 3 to 11 mm long,
cylindrical, brown, glabrous and stemmed. The pappus is in a number of rows and
consists of long, glossy white hairs, which are much lonser than the fruit.
LEAVES, STEM AND ROOT: The plant is a
perennial, 10 to 30 cm high. It has a broadly branched, underground shoot and
root system with a thin round, scaly base. There is also an up to 1.8 m long,
far-reaching, creeping shoot. The flower stern is a scaly, round, tomentose
scape covered with lanceolate, reddish scales, which is 30 cm long when the
fruit ripens. The leaves, which appear after flowering, are basal, coriaceous, cordate-round,
angular, irregularly dentate, long-petioled and tomentose beneath. The leaves
can reach a diameter of up to 30 cm.
CHARACTERISTICS: The taste and
texture is slimy-sweet and the leaves have a honey-like smell when they are
rubbed.
HABITAT: The plant grows
wild in most of Europe, central, western and northern Asia. It has spread to
the mountains of northern Africa and has been introduced into North America.
PRODUCTION: Colt's Foot
flower consists of the fresh or dried flowers of Tussilago farfara. Colt's Foot
herb consists of the fresh or dried, above-ground parts of Tussilago farfara. Colt's
Foot root consists of the fresh or dried, below-ground parts of Tussilago
farfara.
NOT TO BE CONFUSED WITH: The leaves of
various Petasites species, but petasine and flavonoids can be identified using thin
layer chromatography.
SPECIES (FAMILY)
Tussilago farfara L.
(Asteraceae/Compositae) +
SYNONYM(S)
Farfara
ORIGIN
Coltsfoot is a perennial found
in Europe; the United States; Canada; and central, western and northern Asia.
PHARMACOPODIAL AND OTHER MONOGRAPHS
BHC 1992(G6)
BHP 1983(G7)
Complete German Commission E(G3)
Martindale 35th edition(G85)
LEGAL CATEGORY (LICENSED PRODUCTS)
GSL(G37)
CONSTITUENTS
The
following is compiled from several sources, including General Reference G2.
Acids Caffeic
acid, caffeoyltartaric acid, ferulic acid, gallic acid, p-hydroxybenzoic acid,
and tannic acid (phenolic); malic acid and tartaric acid (aliphatic).(1)
Alkaloids Pyrrolizidine-type.
Senkirkine 0.015% and senecionine (minor) (unsaturated)(2, 3) and tussilagine
(saturated).(4)
Carbohydrates Mucilage
(water-soluble polysaccharides) 7–8% yielding various sugars following
hydrolysis (e.g. arabinose, fructose, galactose, glucose, uronic acid and
xylose); inulin (polysaccharide).(5)
Flavonoids Flavonols
(e.g. kaempferol, quercetin) and their glycosides.(1)
Tannins Up
to 17% (type unspecified).
Other Constituents Bitter (glycoside),
choline, paraffin (fatty acid), phytosterols (sitosterol, stigmasterol,
taraxasterol), triterpene (amyrin), tussilagone (sesquiterpene)(6) and volatile
oil.
CHEMICAL COMPONENTS
COMPOUNDS: COLT'S FOOT FLOWER
Mucilages (7%): acidic
polysaccharides
Tannins
Triterpenes: including
beta-amyrin, arnidiol. faradiol
Steroids: including
beta-sitosterol
Pyrrolizidine
alkaloids (traces, not in plants from all places of origin): tussilagine,
isotussilagine, senkirkine. senecionine
Flavonoids
COMPOUNDS: COLT'S FOOT HERB
Mucilages (8%):
acidic polysaccharides
Tannins (5%)
Triterpenes: including
alpha-amyrin, beta-amyrin
Steroids: including
beta-sitosterol, campesterol
Pyrrolizidine
alkaloids (not in plants from all places of origin): senkirkine (0.01%),
senecionine. tussilagine, isotussilagine
Flavonoids
COMPOUNDS: COLTS FOOT ROOT
The roots have
not been fully investigated. Only the presence of triterpenes and sterols has
been established.
COMPOUNDS: COLT'S
FOOT LEAF
Mucilages (8%): acidic
polysaccharides
Tannins (5%)
Pyrrolizidine
alkaloids (traces, not from all sources): tussilagine, isotussilagine,
senkirkine 0.01%), senecionin
Steroids: including
beta-sitosterol, campesterol
Triterpenes: including alpha-
and beta-amyrin
Flavonoids
USES
USES
Coltsfoot
is used to treat respiratory conditions such as bronchitis, cough, and asthma.
It is also used to treat infl ammation of the oral mucosa.
INVESTIGATIONAL USES
Research is underway concerning coltsfoot as an
antimicrobial.
FOOD USE
Coltsfoot
is not commonly used as a food but it is listed by the Council of Europe as a
source of natural food flavouring (category N4). This category indicates that although
coltsfoot is permitted for use as a food flavouring, there are insufficient
data available for an assessment of toxicity to be made.(G16)
HERBAL USE
Coltsfoot
is stated to possess expectorant, antitussive, demulcent and anticatarrhal
properties. It has been used for asthma, bronchitis, laryngitis and pertussis.(G2,
G7, G49,G64)
Figure 1. Coltsfoot (Tussilago farfara).
Figure 2. Coltsfoot –
dried drug substance (leaf).
ACTIONS
Two studies have demonstrated the ability of coltsfoot
to inhibit nitric oxide synthesis in macrophages. The clinical signifi cance of
this fi nding is unknown, however (Ryu et al, 1999). Another study found that
coltsfoot inhibits the binding of both platelet activating factor and Ca2+ entry
blocker to membrane vesicles (Hwang, 1987). Other studies have focused on the
toxic effects of Tussilago farfara L. and the isolation of new chemical
components (Sperl et al, 1995; Wang et al, 1989; Liu et al, 2006). The screening
of 16 medicinal plants showed that 6 possessed signifi cant antimicrobial action
(Kokoska et al, 2002; Kim et al, 2006).
PHARMACOLOGICAL ACTIONS
IN VITRO AND ANIMAL STUDIES
Antibacterial activity has been documented for coltsfoot against
various Gram-negative bacteria including Staphylococcus
aureus, Proteus hauseri, Bordetella pertussis, Pseudomonas aeruginosa and Proteus vulgaris.(7–9)
Anti-inflammatory activity comparable to that of indometacin, determined
in Selye's experimental chronic inflammation test, has been attributed to
water-soluble polysaccharides in coltsfoot.(10) Weak acute anti-inflammatory
activity has been reported for coltsfoot when tested against
carrageenan-induced rat pawoedema.(11, 12)
Platelet-activating factor (PAF) is known to be involved in various
inflammatory, respiratory and cardiovascular disorders. The aggregating action of
PAF is known to be weaker if intracellular concentrations of calcium are low. A
sesquiterpene, L-652469, isolated from coltsfoot buds has been reported to be a
weak inhibitor of both PAF receptor binding and calcium channel blocker binding
to membrane vesicles.(13) This combination of actions was found to effectively
block PAF-induced platelet aggregation. L-652469 was also found to be active
orally, inhibiting PAF-induced rat paw oedema.(13) Interestingly, L-652469 was
reported to interact with the cardiac calcium channel blocker receptor complex
(dihydropyridine receptor), but was also found to be a calcium channel blocker.(13)
Tussilagine has been reported to be a potent cardiovascular and respiratory
stimulant.(6, 14) Dose-dependent pressor activity following intravenous
injection has been observed in the cat, rat and dog.(14) The pressor effect is stated
to be similar to that of dopamine, but without tachyphylaxis. A significant
stimulation of respiration was also observed.(6) Cardiovascular and respiratory
effects are thought to be mediated by peripheral and central mechanisms,
respectively.(6)
CLINICAL STUDIES
There
is a lack of clinical research assessing the effects of coltsfoot and rigorous
randomised controlled clinical trials are required.
ACTIVITIES
Antiaggregant
(1; APA; CAN); Antibacterial (1; CAN; CRC; PH2); Anticholinergic (f; CRC);
Antiedemic (1; CAN; HH2); Antihistaminic (f; CRC; FAD); Antiinflammatory (2;
CAN; KOM; PH2); Antiirritant (2; PHR); Antimitotic (2; KOM); Antispasmodic (1; CAN;
CRC; HH2); Antitussive (1; CAN; CRC; DAA); Calcium Antagonist (1; CAN); Callus-Promoter
(2; KOM); Carcinogenic (1; APA; CRC; PH2); Cardiotonic (1; CAN); CNS-Depressant
(1; DAA); Collyrium (f; CRC); Demulcent (1; CAN; CRC; FAD; PH2); Diaphoretic
(f; CRC; MAD; PIP); Diuretic (f; CRC; PIP); Emollient (f; CRC); Expectorant (1;
CAN; CRC; FAD); Fumitory (f; PH2); Hemostat (f; CRC); Hepatotoxic (1; APA; CAN;
FAD; PH2); Hypertensive (1; APA); Immunostimulant (1; CAN); Pectoral (f; CRC;
MAD); Phagocytotic (1; CAN); Respirotonic (1; CAN); Tonic (f; CRC); Vulnerary
(1; PIP).
INDICATIONS
Adenopathy
(f; PHR; PIP); Ague (f; CRC); Anorexia (F; MAD); Apoplexy (f; CRC; DAA); Asthma
(1; APA; CAN; GMH; PHR); Bacteria (1; CAN; CRC; DAA; PH2); Bleeding (f; CRC);
Bronchosis (2; CAN; FAD; KOM; PH2); Cancer (f; CRC); Cancer, liver (f; JLH);
Cancer, lung (f; CRC; LMP); Carbuncle (f; HAD); Catarrh (2; CAN; CRC; GMH;
KOM); Cold (2; CRC; PIP); Congestion (f; CRC; FAD; LMP); Cough (2; FAD; GMH;
KOM; PH2; PIP); Cramp (1; CAN; CRC; HH2); Diarrhea (f; CRC; POP); Dyspepsia (f;
CRC); Dysphagia (f; DAA); Edema (1; HH2); Emphysema (f; HH2); Enterosis (f;
FEL); Erysipelas (f; GMH; MAD); Escherichia (f; HH2); Fever (f; CRC; DAA; MAD;
PIP); Flu (f; CRC; DAA; LMP; MAD; PHR); Fistula (f; HAD); Gastrosis (f; CRC;
FEL); Headache (f; CRC; FEL); Hematemesis (f; HAD); Hemoptysis (f; CRC; DAA;
LMP); Hoarseness (2; APA; KOM; MAD; PIP); Immunodepression (1; CAN); Induration
(f; CRC; JLH); Infection (1; CRC); Inflammation (2; CAN; FAD; KOM; PH2); Laryngosis
(1; CAN; FEL); Low Blood Pressure (1; APA); Mucososis (2; CRC; FAD; KOM; PH2);
Neurosis (f; CRC); Nicotinism (f; PH2); Ophthalmia (f; CRC); Pertussis (f; CAN;
FEL); Pharyngosis (2; KOM; PH2; PIP); Phthisis (f; CRC; DAA); Plethora (f;
CRC); Pleurosis (f; MAD); Pulmonosis (f; CRC; FAD); Respirosis (2; KOM; 2;
PIP); Rheumatism (f; CRC; PH2); Rhinosis (f; CRC; FEL); Scrofula (f; CRC; FEL;
GMH); Sinusosis (f; CRC); Sore Throat (f; PHR; PIP); Stomatosis (2; APA; PHR;
PH2; PIP); Swelling (1; CAN; CRC; HH2; MAD); Tonsillosis (f; PHR; PIP); Tracheosis
(f; MAD); Tuberculosis (f; CRC; DAA; DEM; MAD); Tumor (f; CRC); Wart (f; MAD);
Water Retention (f; CRC; PIP).
INDICATIONS AND
USAGE
COLT'S FOOT FLOWER. HERB, AND ROOT
UNPROVEN USES: When added to
Colt's Foot leaf, the flower, herb, and root are used to treat rheumatism.
COLT'S FOOT LEAF'
Approved by
Commission E:
• Cough
• Bronchitis
• Inflammation
of the mouth and pharynx
UNPROVEN USES: Colt's Foot leaf
is used for inflammation of the oraFand pharyngeal mucosa. In addition,
cigarettes made of the leaves are used to help cure smoking addiction.
PRODUCT AVAILABILITY
Dried Herb, Extract, Syrup, Tea,
Tincture
PLANT PARTS USED: Dried Flowers, Leaves, Roots
DOSAGES
DOSAGES
·
Adult PO decoction: 0.6-2.9 g
dried herb
·
Adult PO dried herb: 4.5-6
g/day (Blumenthal, 1998)
·
Adult PO fl uid extract: 0.6-2
ml tid (1:1 dilution in alcohol 25% concentration)
·
Adult PO syrup: 2-8 ml tid (1:4
dilution)
·
Adult PO tea: 1-3 tsp dried
herb in 8 oz boiling water, let stand 10 min, strain, take tid
DOSAGES
Dosages
for oral administration (adults) for traditional uses recommended in standard
herbal reference texts are given below.
·
Dried Herb 0.6–2.0 g by decoction three times
daily.(G6)
·
Liquid Extract 0.6–2.0 mL (1 : 1 in 25%
alcohol) three times daily.(G7)
·
Tincture 2–8mL
(1 : 5 in 45% alcohol) three times daily.(G7)
·
Syrup 2–8mL
(liquid extract 1 : 4 in syrup) three times daily.(G7)
DOSAGES
·
2 tsp powdered leaf/cup water (APA; WIC); 0.3–0.6 g solid leaf
extract (PNC); 2–4 ml liquid leaf extract (PNC);
·
4.5–6 g leaf, 0.6–2.0 ml liquid extract (1:1 in 25% ethanol) 3 ×/day
(CAN); 0.6–2.0 g herb as tea 3 ×/day (CAN);
·
2–8 ml tincture (1:5 in 45% alcohol) 3 ×/day (CAN); 2–8 ml syrup
(1:4 liquid extract in syrup) 3 ×/day (CAN);
·
4 g root as diaphoretic (MAD); 1.5–2.5 g leaf or flower/cup tea,
to 6 g day (PH2); 0.6–2 ml liquid flower extract (PNC).
DOSAGES
COLT'S FOOT FLOWER, HERB, AND ROOT
MODE OF ADMINISTRATION: The drug is used
internally through the use of tea and standardized remedies.
PREPARATION: To prepare a
tea, add l .5 to 2.5 gm cut drug to boiling water, then strain after 5 to 10
minutes.
STORAGE: Protect the drug
from light and store it tightly sealed.
COLT'S FOOT LEAF
MODE OF ADMINISTRATION: Whole, cut, and
powdered drug used in teas, infusions, extracts, and tinctures.
PREPARATION: To make an infusion,
pour hot water over 1.5 to 2.5 gm of drug and allow to draw for 10 minutes.
Other preparations are made as follows: liquid extract: 1:1 with 20% ethanol;
extract: 1.1 with 25% ethanol; tincture: 1:5 with 45% ethanol.
DAILY DOSAGE: The total daily
dose is 4.5 to 6 gm of drug. The maximum daily dosage must not be more than 1
meg of total pyrrolizidine alkaloids with 1.2 unsaturated necine structure. The
tea is given several times a day. The dosage for the extract is 2 ml 3 times
daily; for the tincture, it is 8 ml 3 times daily.
STORAGE: Protect the drug
from light and store it tightly sealed.
PRECAUTIONS AND ADVERSE REACTIONS
COLT'S FOOT FLOWER, HERB, AND ROOT
Because of the
possible hepatotoxic and carcinogenic pyrrolizidine alkaloid content, the
administration of the blossoms should be avoided.
COLT'S FOOT LEAF
Colt's Foot
leaves may no longer be brought into circulation in Austria. In Germany, dosages
cannot exceed an intake of 10 meg pyrrolizidine alkaloids with l .2-unsaturated
necic parent substances in the form of tea mixtures, and an intake of l meg in
the form of extracts. Because even traces of the alkaloids present some danger,
one should forgo any administration of the drug.
CONTRAINDICATIONS, INTERACTIONS, AND SIDE EFFECTS
CLASS 2B, 2D (FLOWER); LONGTERM USE
DISCOURAGED. 2B, 2C, 2D (LEAF); do not exceed
recommended dose; not for long-term use
(AHP). Commission E reports flower, herb, root not permitted for therapeutic
use. Contains hepatotoxic
pyrrolizidine alkaloids (PAs) in all plant parts. Leaf is permitted for oral
use. Contraindications in pregnancy
and lactation. CAN cautions that the PAs are genotoxic, carcinogenic, and
hepatotoxic. Because of the PAs, coltsfoot use in pregnancy and lactation is to
be avoided (CAN). Dosage maximum 10 g PA/day (herbal tea) or maximum 1 g PA/day
(extracts, expressed sap) for maximum 4–6 weeks/year (AEH). Commission E
advises not to take more than 4 to 6 weeks of the year at 4.5 to 6 g/day. This
is the only herb (1.5–6 g leaf/day) except related Petasites with toxic PAs
still tolerated by Commission E. Still, CAN cautions that coltsfoot is
phototoxic in guinea pig skin. In guinea pig sensitization experiments, it
showed weak allergenic capacity, possibly due to the sesquiterpene lactones
present in the plant. PAs are toxic to humans, with liver damage with cirrhosis
and ascites, or seneciosis, or veno-occlusive disease (VOD) reported in almost
all cases of severe or fatal intoxications, from intakes of 0.5 mg/kg to 3.3
mg/kg (AEH1). Effective July 1996, the AHP Board of Trustees recommends that
all products with botanical ingredient(s) that contain toxic PAs, including Borago
officinalis, display the following cautionary statement on the label, “For external
use only. Do not apply to broken or abraded skin. Do not use when nursing”
(AHP). Canadians do not allow in food (Blackburn, 1993). Bisset says there is
no danger of acute poisoning when used as prescribed (Bisset, 1994).
Hepatotoxicity of coltsfoot may be due to senkirkine (~150 ppm), highlighting
the dangers of chronic exposure to even low doses of PAs. Rats fed more than 4%
coltsfoot in their diet develop hepatic tumors. Newborn rats are more
susceptible than weanlings to hepatotoxicity of senkirkine despite lacking the
hepatic microsomal enzymes required to produce the toxic pyrrholic metabolites.
Fatal hepatic venoocclusive disease
was documented in a newborn infant whose mother chronically consumed herb teas
during pregnancy (coltsfoot and senecio specified). The mother exhibited no
signs of hepatic damage again
suggesting increased sensitivity of the fetal liver to PA toxicity. Animal studies document placental
transfer and secretion into breast milk of unsaturated Pas (CAN). Excessive doses may interfere with blood pressure and heart therapy (CAN).
CONTRAINDICATIONS AND PRECAUTIONS
COLT'S FOOT
FLOWER. HERB, ROOT. AND LEAF
Administration
during pregnancy and while nursing is contraindicated.
CONTRA-INDICATIONS,
WARNINGS
In
view of the known pyrrolizidine alkaloid content, excessive or prolonged
ingestion should be avoided. In particular, herbal teas containing coltsfoot
should be avoided.
Drug Interactions None
documented. However, the potential for preparations of coltsfoot to interact
with other medicines administered concurrently, particularly those with similar
or opposing effects, should be considered. There is limited evidence from
preclinical studies that tussilagine, a constituent of coltsfoot, has pressor activity.
However, the clinical significance of this, if any, is not known.
Pregnancy and Lactation Coltsfoot should not be taken during pregnancy or
lactation in view of the toxicity associated with the pyrrolizidine alkaloid
constituents. Coltsfoot is reputed to be an abortifacient.(G30)
CONTRAINDICATIONS
CLASS
2B/2C/2D HERB (FLOWERS).
Coltsfoot should not be used during pregnancy and breastfeeding.
It should not be given to children. Coltsfoot should not be used by persons
with hepatic disease or those who are hypersensitive to ragweed, chamomile, or
other members of the composite family. Persons with cardiac disease, or
hypertension should use this herb cautiously. Coltsfoot should not be used for
longer than 6 weeks. Pyrrolizidine alkaloid content should not exceed 10 mcg.
SIDE EFFECTS/ADVERSE REACTIONS
CNS: Fever
CV: Hypertension
GI: Nausea,
vomiting, anorexia, diarrhea, jaundice, hepatotoxicity (rare)
INTEG: Hypersensitivity
reactions
RESP: Upper
respiratory infection
INTERACTIONS
Drug
Antiarrhythmics, antihypertensives: Coltsfoot may antagonize antiarrhythmics and
antihypertensives; avoid concurrent use (theoretical).
Herb
Eucalyptus: Eucalyptus
may increase the toxicity of coltsfoot; avoid concurrent use (theoretical)
(Jellin et al, 2008).
Pyrrolizidine alkaloid (UPA)-containing herbs (borage, gravel root, agrimony, petasities, comfrey,
dusty miller, ragwort): Use
with these herbs and coltsfoot will lead to increased toxicity; do not
use concurrently (Jellin et al, 2008).
Lab Test
AST, ALT, alkaline phosphatase: Coltsfoot may increase these levels.
EFFECTS
EFFECTS: COLT'S FOOT LEAF
The pyrrolizidine alkaloids are
antibacterial, carcinogenic, and hepatotoxic. The mucin polysaccharides cause a
demulcent, sequestering, and anti-inflammatory effect. In animal experiments
there was evidence of a stimulating effect on the ciliated epithelium.
EFFECTS: COLT'S FOOT FLOWER, HERB, AND ROOT
The mucin contained in the drug has a
sequestering effect and envelopes the mucous membrane with a layer that protects
the throat from chemical and physical irritation and thereby reduces cough
irritation. The pyrrolizidine alkaloids are antibacterial, carcinogenic, and
hepatotoxic.
SIDE-EFFECTS, TOXICITY
There is a lack of
clinical safety and toxicity data for coltsfoot and further investigation of
these aspects is required. Coltsfoot has been reported to be phototoxic in
guinea-pig skin.(15) Pyrrolizidine alkaloids with an unsaturated pyrrolizidine nucleus
are known to be hepatotoxic in both animals and humans (see Comfrey). Of the
pyrrolizidine alkaloids documented for coltsfoot, senecionine and senkirkine
are unsaturated. Chronic hepatotoxicity has been described in rats following
the incorporation of coltsfoot into their diet at concentrations ranging from 4–33%.(16)
After 600 days, it was found that rats fed more than 4% coltsfoot had developed
hepatic tumours (haemangioendothelial sarcoma) while none were observed in the
control group.
Furthermore, histological
changes associated with pyrrolizidine alkaloid toxicity, such as centrilobular
necrosis of the liver and cirrhosis, were observed in many of the rats who had
ingested coltsfoot but who had not developed tumours.(16) The hepatotoxicity of
coltsfoot was attributed to senkirkine, which is present at a concentration of
only 0.015%, thus highlighting the dangers associated with chronic exposure to
low concentrations of pyrrolizidine alkaloids.
Newborn rats have been found
to be more susceptible than weanlings to the hepatotoxic effects of senkirkine
despite lacking the hepatic microsomal enzymes required for the formation of
the toxic pyrrolic metabolites.(17) Fatal hepatic veno-occlusive disease has
been documented in a newborn infant whose mother had regularly consumed a
herbal tea during pregnancy.(18) Analysis of the herbal tea revealed the
presence of 10 different plants including coltsfoot and a Senecio species
(known source of pyrrolizidine alkaloids, see Liferoot). The mother exhibited
no signs of hepatic damage, suggesting an increased sensitivity of the fetal
liver to pyrrolizidine alkaloid toxicity.
Pre-blooming coltsfoot
flowers are reported to contain the highest concentration of alkaloids.(3) Considerable
loss of both senkirkine and senecionine has been observed upon prolonged storage
of the dried plant material.(3) Senkirkine and senecionine are both easily
extracted into hot water and, therefore, would presumably be ingested in a herbal
tea prepared from the fresh plant.(3) A cup of tea prepared from 10 g
pre-blooming flowers has been estimated to contain a maximum of 70 mg
senecionine and 1.4 mg senkirkine. Tea from the young leaves or mature plant would
presumably contain considerably lower concentrations of alkaloids.(3) These
concentrations are not considered to represent a health hazard compared to the
known hepatotoxicity of senecionine (intravenous LD50 64 mg/kg body weight,
mice).(3)
However, prolonged
exposure to low concentrations of pyrrolizidine alkaloids has resulted in
hepatotoxicity (see Comfrey). Tussilagine LD50 (mice, intravenous injection)
has been determined as 28.9 mg/kg.(14)
CLIENT CONSIDERATIONS
ASSESS
·
Assess the reason the client is
using coltsfoot.
·
Assess for hypersensitivity
reactions. If these are present, discontinue the use of this herb and
administer antihistamines or other appropriate therapy.
·
Assess for hepatotoxicity
(increased hepatic function tests, jaundice, clay-colored stools, right
upper-quadrant pain). If these occur, herb use should be discontinued.
·
Assess for the use of antiarrhythmics
and antihypertensives (see Interactions).
ADMINISTER
·
Instruct the client to store
coltsfoot products in a cool, dry place, away from heat and moisture.
·
Because of the presence of
hepatotoxic pyrrolizidine alkaloids, caution the client not to use coltsfoot
for longer than 6 weeks.
TEACH CLIENT/FAMILY
·
Caution the client not to use
coltsfoot in children or those who are pregnant or breastfeeding because
hepatotoxicity may occur.
·
Advise the client to report any
side effects to the provider.
·
Caution the client not to
confuse peppermint with coltsfoot; they are similar in appearance.
PREPARATIONS
PROPRIETARY MULTI-INGREDIENT PREPARATIONS
Argentina:
Arceligasol; Negacne. Czech Republic: Perospir; Species Pectorales Planta. Italy:
Lozione Same Urto. Spain: Llantusil. UK: Antibron; Chesty Cough Relief.
REFERENCE
Barnes, J., Anderson, L. A., and Phillipson, J. D. 2007. Herbal
Medicines Third Edition. Pharmaceutical Press. Auckland and
London.
Duke, J. A. with Mary Jo Bogenschutz-Godwin, Judi duCellier, Peggy-Ann K.
Duke. 2002. Handbook of Medicinal Herbs 2nd Ed. CRC Press
LLC. USA.
Gruenwald, J., Brendler,
T., Jaenicke, Ch. 2000. PDR for Herbal
Medicines. Medical Economics Company, Inc. at Montvale, NJ
07645-1742. USA
Linda S-Roth. 2010. Mosby’s Handbook Of Herbs & Natural
Supplements, Fourth Edition. Mosby Elsevier. USA
No comments:
Post a Comment